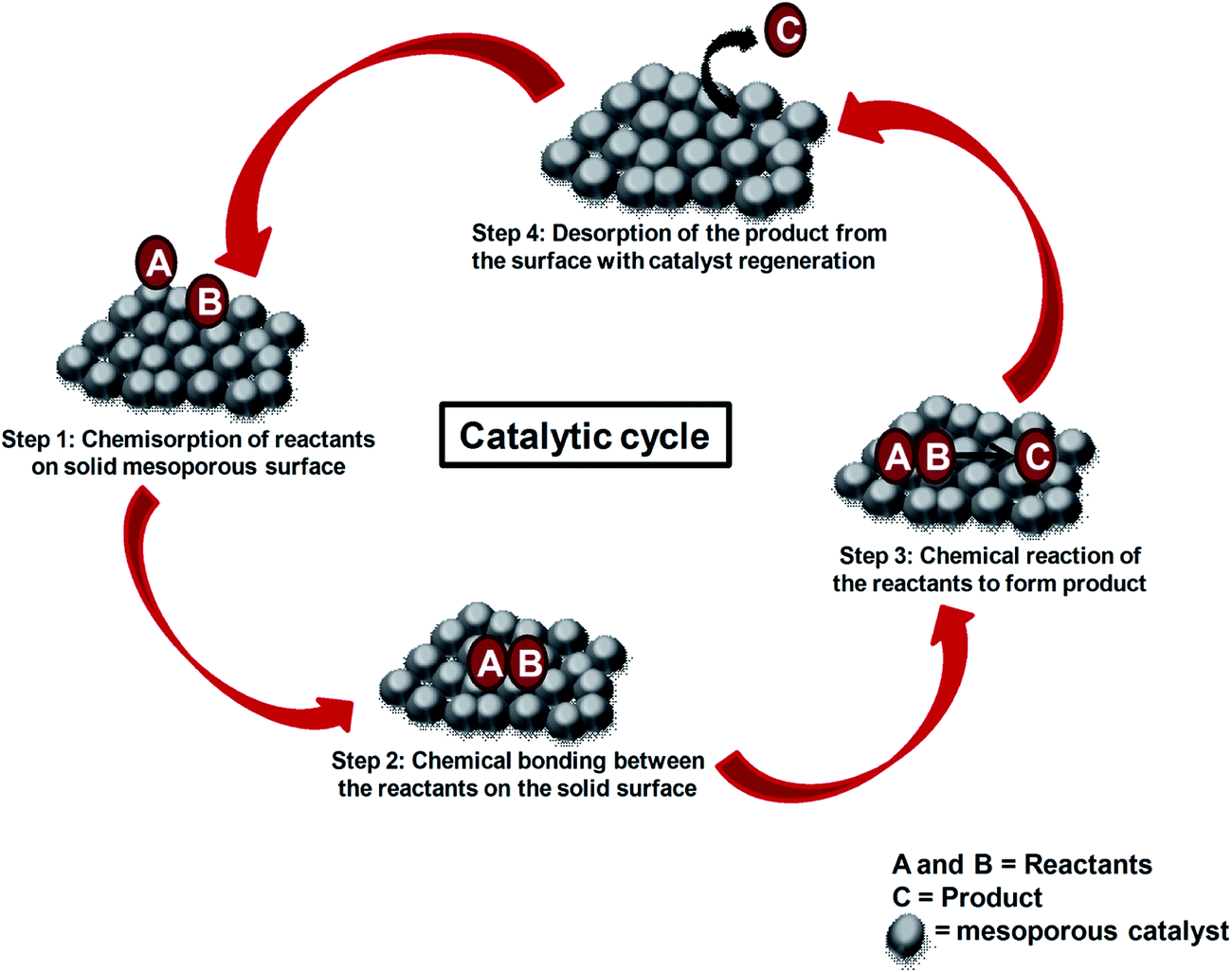
This Catalysis 2019 is the International platform which brings together the collection of investigators who are at the forefront in the field of chemistry, catalysis and chemical engineering. The scientific program will include oral presentations of subdisciplines, keynote sessions led by eminent scientists and poster sessions presented. Intermetallic catalysts for water splitting and petroleum reforming Complex metallic alloys (CMAs) have been long viewed as crystallographic curiosity rather than applied materials. 1 The situation has been changing over the last decade, as the advanced imaging instrumentation and more powerful computational techniques afforded deeper insight into the structure. According to Surface adsoprtion theory heterogeneous catalysis has five stages: Stage 1: Diffusion of Reactant(s) to the Surface: The rate at which reactants will diffuse to the surface will be influenced by their bulk concentration and by the thickness of the boundary layer. In this video Ill teach you about catalysts. Specifically, Ill explain the difference between a homogeneous catalyst and a heterogeneous catalyst, with examples of each. Convenor: Annette Trunschke New developments in heterogeneous catalysis in areas of application such as biomass utilisation, clean hydrogen production and CO2 utilisation. In addition new areas of synthesis and materials including single atom catalysis, MOFs and advanced characterisation methods Heterogeneous Catalysis is recommended for both students and professionals in catalysis science and materials science. All readers will benefit from the experience and wisdom of Eli Ruckenstein, one of the most esteemed scientists in the world today. This book is based on a graduate course and suitable as a primer for any newcomer to the field, this book is a detailed introduction to the experimental and computational methods that are used to study how solid surfaces act as catalysts. The Basis and Applications of Heterogeneous Catalysis (Oxford Chemistry Primers) May 14, 1998. 07 (35 used new offers) Metal Oxides in Heterogeneous Catalysis Jan 27, 2018. He continued the study of heterogeneous catalysis as a research associate at the same place. In 1989 he did a postdoctoral work with Professor Richard G. In 1990, he returned to Japan as an Associate Professor at Catalysis Research Center, Hokkaido University. The development of heterogeneous catalysts for green chemical synthesis is a growing area in catalysis and greensustainable chemistry. This review summarizes recent examples of hydrogen transfertype reactions using supported transition metal catalysts with special emphasis on the direct synthesis of chemicals by borrowing hydrogen methodology. We welcome you to the third edition on Catalysis Conference 2019 cohosted by topics on biocatalysis conference and heterogeneous catalysis conference and events is ultimate multidisciplinary meeting space for scientists to network, grow and share their cuttingedge research in chemistry. Heterogeneous catalysis is one of the cornerstones of chemical industry, and more efficient catalysts are key in making the world more energy efficient and less polluting. An example of heterogeneous catalysis is the use of finely divided platinum to catalyze the reaction of carbon monoxide with oxygen to form carbon dioxide. This reaction is used in catalytic converters mounted in automobiles to eliminate carbon monoxide from the exhaust gases. In heterogeneous catalysis the overall reaction consists of a sequence of consecutive steps, such as diffusion, adsorption of reactants and products, surface reaction and desorption of the products. The heterogeneous reactions take place on the catalyst surface where some adsorbed species are present during the reaction. In chemistry, heterogeneous catalysis refers to the form of catalysis where the phase of the catalyst differs from that of the reactants. Phase here refers not only to solid, liquid, vs gas, but. Catalysis, Catalysts, Heterogeneous Catalysis, Copper Nickelantimony nanoparticles confined in SBA15 as highly efficient catalysts for the hydrogenation of nitroarenes NiSb nanoparticles confined in SBA15 are found to be highly efficient catalysts for nitroarene reduction reactions. In heterogeneous catalysis, the reactants adsorb onto binding sites on the surface of the catalyst, and the availability of these reaction sites can limit the rate of heterogeneous reactions. catalyst: A substance that increases the rate of a chemical reaction without being consumed in the process. 1 I Introduction Catalysis is a term coined by Baron J. Berzelius in 1835 to describe the property ofsubstances that facilitate chemical reactions without being. Our textbook Catalysis: Concepts and Green Applications (2nd edition, 2017, ISBN ) covers homogeneous, heterogeneous and biocatalysis from a Green Chemistry perspective. A WileyVCH bestseller, it is the most popular introductory textbook in catalysis. Track 1: Homogeneous catalysis, Molecular Catalysis In chemistry, homogeneous catalysis will be catalysis in a solution by a solvent catalyst. Entirely, homogeneous catalysis alludes to catalytic reactions where the catalyst is in same stage from the reactants. Homogeneous catalysis applies to reactions in the gas stage and even in solids. The Impact of Nanoscience on Heterogeneous Catalysis Alexis T. Bell Division of Chemical Sciences, Lawrence Berkeley National Laboratory, and Department of Chemical Engineering, University of California, Berkeley, CA, USA. In photocatalysis light is absorbed by the catalyst or a reactant during the reaction. This can take place in ahomogeneous or heterogeneous system. Today's top 18 Heterogeneous Catalysis jobs in United States. Leverage your professional network, and get hired. New Heterogeneous Catalysis jobs added daily. Heterogeneous catalysis plays a part in the production of more than 80 of all chemical products. It is therefore essential that all chemists and chemical engineers have an understanding of the fundamental principles as well as the applications of heterogeneous catalysts. Then relocate to Shanghai and specialize in heterogeneous catalysis, oversee catalyst evaluation with stateofthe art test units, lab scale catalyst synthesis and catalyst scaleup. Carry out challenging RD projects to serve internal and external customers and propose new catalyst and. Metal species with different size (single atoms, nanoclusters, and nanoparticles) show different catalytic behavior for various heterogeneous catalytic reactions. It has been shown in the literature that many factors including the particle size, shape, chemical composition, metalsupport interaction, and metalreactantsolvent interaction can have significant influences on the catalytic. Heterogeneous catalysis significantly shapes society, as it plays an important part in the production of base chemicals, in the petrol industry and the synthesis of fine chemicals and pharmaceuticals. Engineers A Brief History and Fundamental Catalytic Principles What is Catalysis? The science of catalysts and catalytic We study bioinspired heterogeneous catalysts for the production of renewable energy, fuels, and chemicals Heterogeneous catalysis plays a part in the production of more than 80 of all chemical products. It is therefore essential that all chemists and chemical engineers have an understanding of the fundamental principles as well as the applications of heterogeneous catalysts. Research in the Department of Heterogeneous Catalysis (F. Schth) The department of Heterogeneous Catalysis is now fully in steady state operation, i. while the head of the department, F. Schth, remains in place, group leaders are leaving the Institute to face new challenges at other institutions, and new group leaders start their. For more news about Professor Ozkan and the Heterogeneous Catalysis Lab, please visit the chemical engineering department's website. It is never too early to get started with science! Ellie and Gavin enjoying a day of chemistry experiments in our labs with Dr. Seval Gunduz and Professor Ozkan. SILP technology is not simply heterogeneous, it is a unification of heterogeneous and homogeneous catalysis because inside your support you still have the homogeneous catalyst and I think that is the real great advantage, explains Franke. In catalysis: Heterogeneous catalysis. Many catalytic processes are known in which the catalyst and the reactants are not present in the same phasethat is, state of matter. These are known as heterogeneous catalytic reactions. Catalysis and Catalytic Processes by Dr. Pant, Department of Chemical Engineering, IIT Delhi. Loading Unsubscribe from nptelhrd. 1 What is Heterogeneous Catalysis? 3 The Three Basic Laws of Catalysis 7. 2 Heterogeneous Catalytic Processes 15. 2 Important Heterogeneous Catalytic Reactions and Processes 19. In homogeneous catalysis, the catalyst is dissolved in the reaction mixture. In heterogeneous catalysis, however, the reaction mixture flows over a catalytic load. TIB Chemicals provides copper and nickel compounds for both applications. Heterogeneous Catalysts for Olefin Metathesis. Propylene (C 3 ) is the second largest feedstock for the petrochemical industry and a shortage is developing that will increase over the next decade. To meet this demand, industry is turning to heterogeneous olefin metathesis catalysis via C 2 C 4 2C 3. A key challenge is to improve performance of catalysts for this reaction. types of catalysis This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. You will also find a description of one example of autocatalysis a reaction which is catalysed by one of its products. Journal of Catalysis is the premier scholarly publication in the field of catalysis and an indispensable source of information for chemists and chemical engineers in both industrial and academic fields. Zeolites and zeolitelike microporous materials have been playing an everincreasing role in heterogeneous catalysis. Zeolites are the most important heterogeneous catalyst having huge industrial applications in fields like petrochemistry, petroleum refining, chemical synthesis. Yuriy Roman MIT research group The focus of our research lies at the interface of heterogeneous catalysis and materials design. This book is a fundamental handbook on heterogeneous catalysis. Here the main concepts and meaning of catalytic phenomena are formulated, the notions of the catalytic action nature and general regularities of heterogeneous catalysis are presented, the mechanisms and kinetics of. The work of the group is concentrated on the synthesis and characterization of inorganic materials with an application focus in heterogeneous catalysis. The metrics for this position include heterogeneous catalyst design and synthesis, physical and chemical characterization of new catalysts, screening on The first comprehensive survey of the principles and applications of heterogeneous catalysis! Starting with the invention of Dbereiner's tinder box and reaching importance with Haber's development of ammonia synthesis, heterogeneous catalysis has become a multibillion dollar business. You have expertise in technical chemistry with an indepth understanding of heterogeneous catalysis. Fluency in German and English as well as familiarity with chemical software packages is considered as a. 1 An introduction to kinetics in heterogeneous catalysis How to measure properly activity and selectivity, what are underlying fundamentals, how looks like In heterogeneous catalysis, the diffusion of reagents to the surface and diffusion of products from the surface can be rate determining. A nanomaterialbased catalyst is an example of a heterogeneous catalyst. Analogous events associated with substrate binding and product dissociation apply to homogeneous catalysts..